Select All of the Following Reactions That Are Endothermic
The energy released when making the new bonds is less than the energy required when the bonds are broken making the reaction endothermic by 1 8 0 k J per mole. ½N 2g 32H 2g NH 3g 110 kcalmole.
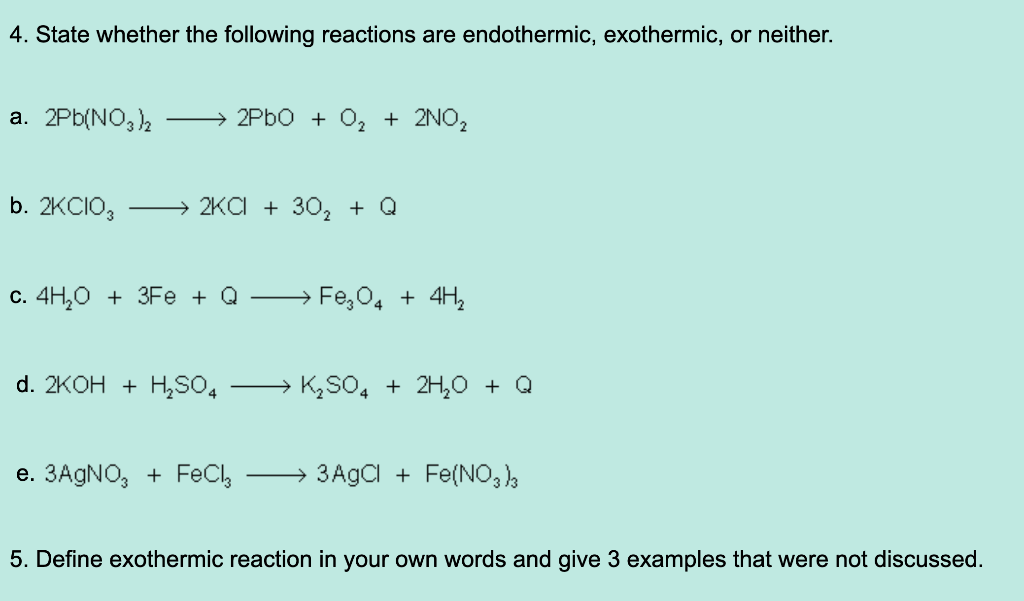
Solved 4 State Whether The Following Reactions Are Chegg Com
½N 2g O 2g 81 kcal NO 2g ½N 2g ½O 2g NOg ΔH 216 kcalmole.

. B Decomposition of vegetable matter into compost. 2CO O2 2CO2 Carbon monoxide and oxygen combine to produce carbon dioxide. Endo means in and thermic means heat.
½N2 g ½O2 g NO g ΔH 216 kcalmole. Select all that apply H 2 g ½O 2 g H 2 Oaq H -5783 kcalmole ½ N 2 g ½O 2 g NOg H 216 kcalmole ½N 2 g O 2 g 81 kcal NO 2 g ½N 2 g 32 H 2 g NH 3 g 110 kcalmole NH 3. Select all that apply Select all that apply N2g 2 Ng 12g 219 CH4g 2 02 - CO2g 2 H2Og NH3g NH31 2 Brg Br2g Submit.
4 Decomposition of calcium carbonate to form quick lime and carbon dioxide. Thus combustion reaction N 2 O 2 2 N O of nitrogen is endothermic. A Raising the temperature increases the rate of an endothermic reaction and decreases the rate of an exothermic reaction.
Consider the reaction given below. The bond energy of each carbon-oxygen bond in carbon. Which of the following reactions is an endothermic reaction.
-½n2 g ½o2 g no g δh 216 kcalmole. Select all of the following reactions that are endothermic. Which of the following are endothermic.
The endothermic reaction is the type of reaction that absorbed the energy from the system while exothermic reaction released energy in the system. C Process of respiration. Potential energy diagram for an endothermic reaction 1.
Select all of the following statements which are true. Which reactions are endothermic. In this reaction CaC O 3 breaks.
NH3 g ½N2 g 32H2 g ΔH 110 kcalmole. Which of the following will increase in concentration if the volume of the reaction vessel containing the reaction is increased 2NOBr g et 2NO g Br2 g Select all that apply NO 9 Br2 g NOBr g There is no concentration change for any of the chemical species. Select all of the following reactions that are endothermic.
The endothermic reactions are those in which heat is needed or added into the system for the reactants to react. CHEMWORK Check the reactions which are endothermic. Which of the following reactions is an endothermic reaction.
Select 3 correct answers Sublimation Fusion Vaporization Deposition Condensation Solidification Expert Solution. CaC O 3 CaO C O 2. The value of ΔH is positive.
Chemical reactions are accompanied by a change in energy mainly in the form of heat. SELECT THE CORRECT ANSWER. No Answer DELTA PONS 11 is Since tuus endothenic reallows AH postque cuaithon Now we cou see B g que m the c 6 in sole Alg less spontando entropy of negative.
The total bond energy of the products is 1472 kJ. Energy Conservation Quick Check 1Is freezing an endothermic or exothermic process. Neutralisation reactions are always exothermicendothermic in nature ___________ of the following are.
½N2 g O2 g 81 kcal NO2 g ½N2 g 32H2 g NH3 g 110 kcalmole. - If the container of a reaction becomes colder during the reaction the reaction is endothermic Calculate deltaH o rxn for the reaction A 2B - 2C using the deltaH o f values given below 2c -. Werene a 4 6 are positive.
A Burning of coal. -½n2 g o2 g 81 kcal no2 g. QUESTION 5 Which of the following is true for an endothermic reaction.
Answer Here Endothermic Reaction. 25072021 0620 keke6361. Dp The reaction shifts to the right when the temperature is decreased.
D Decomposition of calcium carbonate to form quicklime and carbon dioxide. The given system has. Spontaneous at all temperatures.
For the following endothermic reaction Ag B g C g This reaction is. 1- Use the reaction to complete the sentence. - Get the answer to this question and access a vast question bank that is tailored for students.
In these reactions heat is. 1 Burning of coal. Gada this spectroddy produck reaction is this AH 4 -b.
QA Kdecreases with increase in temperature DB K increases with increase in temperature Dc The reaction shifts to the right when the temperature is increased. C Increasing the surface area of the. From the reactions in the choices presented the endothermic reactions are the second third and fifth.
This is an example of decomposition reaction. Ob Raising the temperature increases the rate of an exothermic reaction and decreases the rate of an endothermic reaction. Breaking bonds is endothermic and making new bonds is exothermic.
A reaction in which heat is given out is called exothermic while a reaction in which heat is taken in is endothermic. 2 Decomposition of vegetable matter into compost. Select all that apply.
H 2g ½O 2g H 2Og ΔH -5783 kcalmole. Select all of the following reactions that are endothermic. Chemistry 09012020 0631 MysteryDove12.
-h2 g ½o2 g h2o g δh -5783 kcalmole. H2 g ½O2 g H2O g ΔH -5783 kcalmole. 3 Process of respiration.
The reaction below is endothermic.
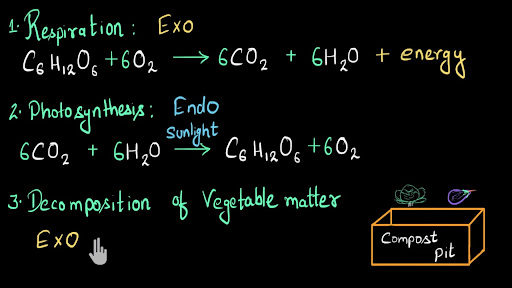
Exothermic And Endothermic Common Processes And Solved Examples Video Khan Academy

Ap Chemistry Experiment 19 Qualitative Determination Of Cations And Anions Lab Chemistry Experiments Ap Chemistry Chemistry

Carbocation Rearrangements In Ring Expansion Reactions Practice Problem Reactions Chemistry The Expanse
0 Response to "Select All of the Following Reactions That Are Endothermic"
Post a Comment